
Are Your Cosmetic Labels Compliant?
Estimated reading time: 5 minutes
What you need to make your beauty & cosmetic labels compliant
Other articles you might like:
Are your cosmetic labels compliant with the requirements of the U.S. Food & Drug Administration (FDA)? Strict guidelines are in place to protect consumers from products that are potentially unsafe or have deceptive labels.
The FDA regulates beauty and cosmetic products; however, it places much of the responsibility on the makers and manufacturers. To guide you through the regulations, we’ve taken a closer look at what you need to ensure your cosmetic labels are compliant. Make sure to check all federal and state regulations before ordering your cosmetic labels.
What are cosmetics?
The Federal Food, Drug & Cosmetic Act (FD&C Act) defines cosmetics as products for use on the human body for cleansing, beautifying, promoting attractiveness, or altering the appearance without affecting the body’s structure or functions. Cosmetics include products such as creams, lotions, perfumes, lipsticks, nail polishes, makeup, shampoos, hair colors, toothpaste, and any material for use as a component of a cosmetic.
Soap products made up mainly of an alkali salt of fatty acid and don’t make any label claim other than the cleansing of the human body are not cosmetics under the law.
Principal Display Panel (PDP)

The principal display panel is the label that is seen under normal conditions for retail sales. The information shown here should include:
- Product identity – What is your product, i.e. soap, lotion, scrub
- Net contents – Weight or volume of product
- Ingredients – What is in your product
- Warning labels – Such as “for external use only”
Additional information not on the PDP label should also be somewhere on the packaging.
- Directions for safe use
- Name and place of business
- Any other required information
According to the Food and Drug Administration (FDA), all the information above must appear on the label of the outer container. This is usually a box, carton, or wrapper that holds an inner or immediate item. The immediate item that holds the actual product is the outer package if there isn’t a box or carton.
For specific details on labeling requirements under US laws and regulations, read the Cosmetics Labeling Guide from the FDA.
Symbols to help make cosmetic labels compliant
So how do you fit all this information on your cosmetic labels? Use the symbols below to communicate more information to your customers on a smaller label or package.
Shelf life
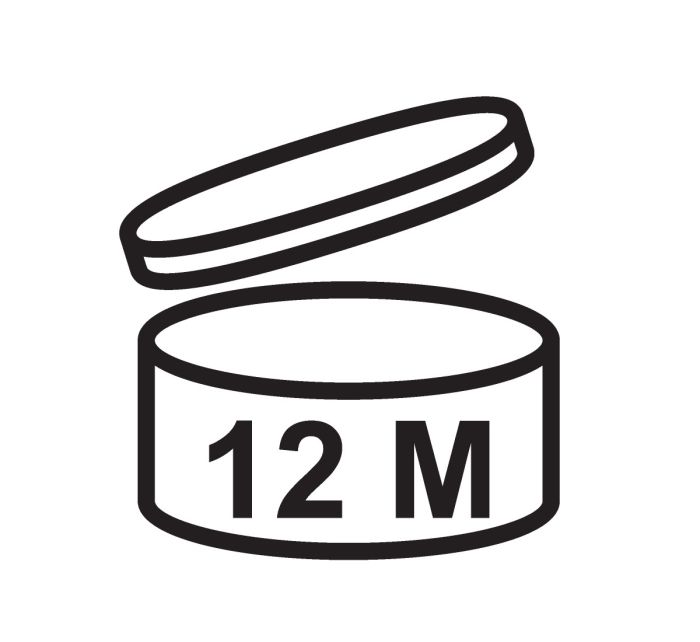
The Period After Opening (PAO) symbol is the open jar icon. This icon indicates how many months the product will be good after it’s opened. This is typically on products with a shelf life of 30 months or more.
Best before date
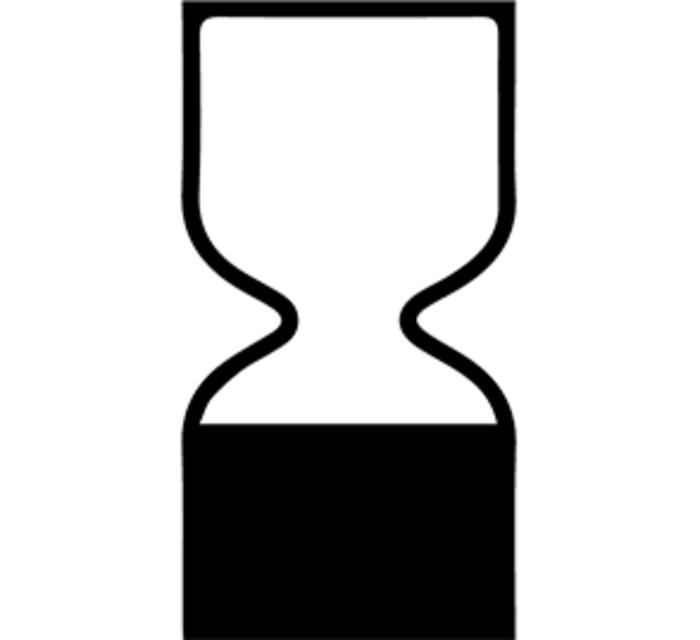
For products with a lifespan of fewer than 30 months, an hourglass icon and a “best before end of,” or BBE date is displayed.
Net contents
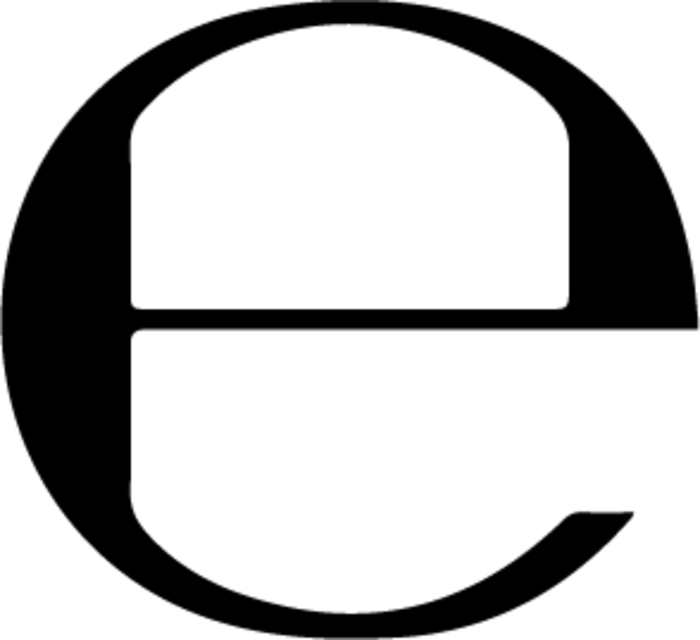
The lowercase “e” is known as the estimated sign or e-mark. It is required by the EU and basically indicates that the quantity of the product in a batch of packages is the same as what’s stated on the label.
Refer to insert

When there’s required information such as ingredients and instructions to provide (and sometimes in multiple languages) there may not be enough room to do so. So this symbol lets consumers know there’s a leaflet, card, or other inserts to refer to inside the packaging.
Responsibility for packaging
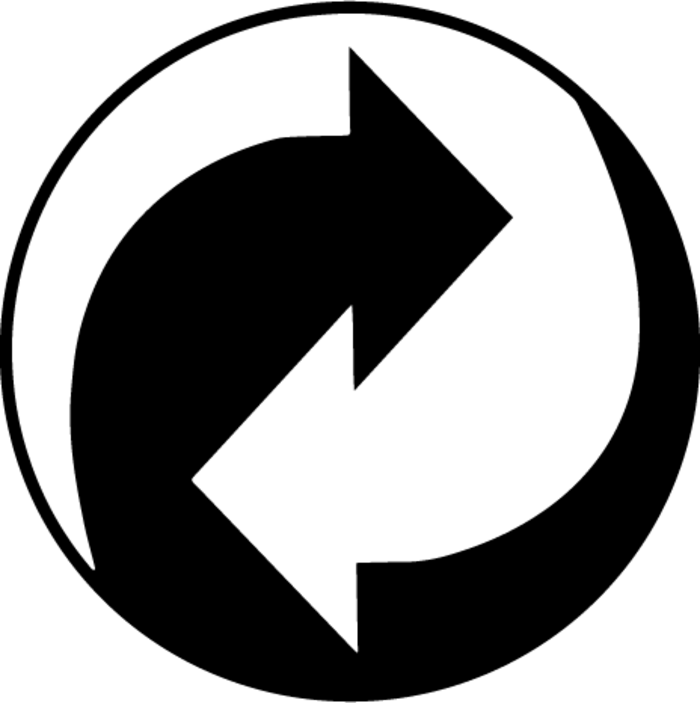
The Green Dot trademark indicates the manufacturer financially contributes to a recycling organization to manage its packaging waste in an ecologically responsible manner. It is not a recycling symbol.
Responsible forest management

The Forest Stewardship Council (FSC) trademark indicates the paper the company uses comes from responsible sources. The use of the logo requires authorization and a license code from the FSC.
Organic seal

The Organic seal indicates the product has met strict production and labeling requirements of the USDA, and the final product is certified. Selling a product as organic when it is not can cost a company up to $11K for each violation.
US Public Health Service seal

Products bearing the U.S. Public Health Service seal must contain 70% organic ingredients. Also, they must follow strict guidelines for manufacturing and processing.
Cruelty-free

PETA runs the well-known Beauty Without Bunnies certification program, however, the Leaping Bunny is another internationally recognized certification that upholds a stricter program and mandatory audits.
Body care standards

Whole Foods developed strict baseline body care standards in quality sourcing and environmental impact in order for products to earn the Premium Body Care logo. They’ve also identified more than 400 ingredients deemed unacceptable.
Full label disclosure

The Environmental Working Group, or EWG, established the EWG VERIFIED mark to indicate the product meets the strictest standards regarding harmful chemicals.
GMO avoidance

To earn the Non-GMO Project Verified seal, products must complete third-party verification for their product labeling and certifications to ensure products have been thoroughly evaluated by an independent party for compliance.
Gluten-free

The Gluten-Free Certification Organization (GFCO) leads a strict gluten-free certification program. They inspect products and manufacturing facilities for the presence of gluten. Products bearing the gluten-free symbol must be certified by a third party to earn certification.
You can print out the chart below for easy reference.

Please note: We are not lawyers or a law firm and we do not provide legal, business, or regulatory advice. Our sites and services are not substitutes for the advice or services of an attorney. We recommend you consult a lawyer or other appropriate professional if you want legal, business, or regulatory advice.



